Rapid electronic detection
Rapid detection of infectious diseases, from Ebola virus disease (EVD) to recent coronavirus disease 2019 (COVID-19) and to future disease X, is crucial to isolating infection and disrupting the transmission. Current biomarker-based diagnostics mainly relies on the detection of the genetic (or molecular), antigenic, or serological (antibody) markers. Genetic detection, typically based on polymerase chain reaction (PCR), is the gold standard but more complex, costly, time-consuming and instrument-demanding. We are developing a novel Nano2RED sensing platform, which utilizes novel signal transduction pathways to convert biological binding into circuit readout, without using fluorescence microscopy or enzymatic amplifications. It has demonstrated its use in detection of Ebola and SARS-CoV-2 antigens, antibodies, and small molecules (cannabinoids). We are currently supported by USDA and NIH to detect African Swine Fever (ASF) and neutralizing antibodies against variants of SARS-CoV-2 viruses, but are also exploring this platform for detection of other diseases, including Lyme disease, Hepatitis C, Shiga toxin-producing E. col, etc. The platform will also be engineered towards different use for its rapid turnaround time and low cost (e.g. tests in resource-limited healthcare settings), small volume requirement (e.g. newborn and infant screening), and/or high accuracy (e.g. early detection of cancer or neurodegenerative diseases).
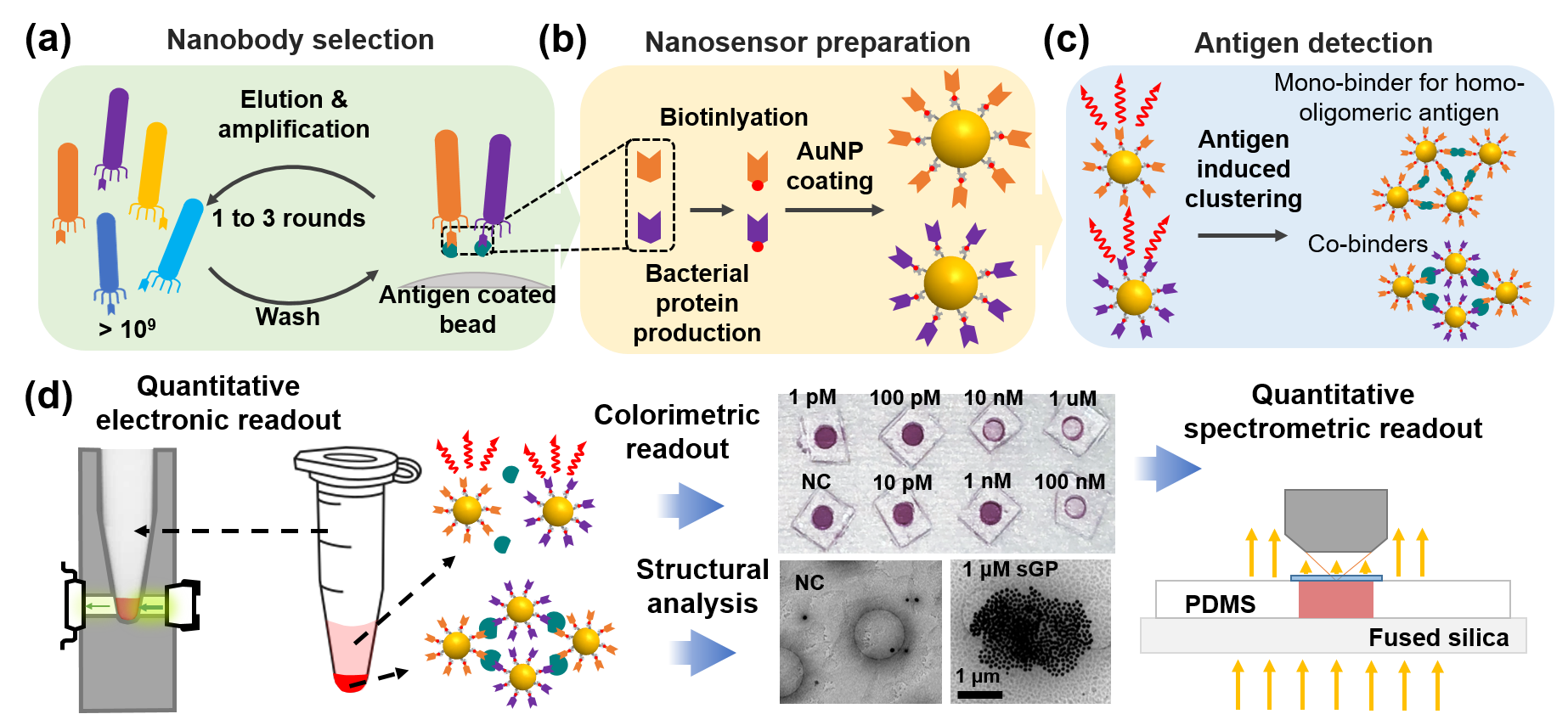
Nano2RED for Ebola virus and small molecule sensing
Using Ebola sGP protein as the target antigen, we synthesized highly specific nanobodies, and demonstrated that sGP-nanobody binding triggers a suspension color change from AuNP aggregation and precipitation. The signals can be read out qualitatively by eyes, or quantitatively using photodetectors with LEDs as a light source. Unlike fluorescence based readout that requires complex optical elements, this electronic readout format can be designed very portable and inexpensive for resource-limited settings. The Nano2RED assay detects sGP proteins in diluted serum down to ~10 pM and 10-100 times better than enzyme-linked immunosorbent assays (ELISA), while specifically distinguishing a membrane-anchored protein isoform (GP1,2). In addition, we have developed a rapid detection scheme to shorten the assay time to a few minutes, by introducing a pre-concentrating step that accelerates antigen-dependent AuNP precipitation. This sensitive, specific, rapid, quantitative, and low-cost platform could be standardized and made available within ~4 weeks for a wide variety of pathogens, potentially well-suited to both clinical and home use for identifying infective individuals, quantifying viral load, and analyzing the immune response.
Reference:
- Xiahui Chen †, Shoukai Kang †, MD Ashif Ikbal †, Zhi Zhao, Yuxin Pan, Jiawei Zuo, Liangcai Gu*, and Chao Wang*, “Synthetic Nanobody-Functionalized Nanoparticles for Accelerated Development of Rapid, Accessible Detection of Viral Antigens,” Biosensors and Bioelectronics, v 202, pp. 113971, 2022, bioRxiv 2021.05.09.443341.
- MD Ashif Ikbal†, Shoukai Kang†, Xiahui Chen, Liangcai Gu*, and Chao Wang*, “Picomolar-Level Sensing of Cannabidiol by Metal Nanoparticles Functionalized with Chemically Induced Dimerization Binders,” ACS Sensors, vol. 8, pp. 4696–4706, 2023. https://doi.org/10.1021/acssensors.3c01758.
Nanoparticle-supported, rapid electronic detection (NaSRED) system design:
Instead of using nanobodies, other binders can also be functionalized on AuNPs for rapid electronic detection. Here conventional monoclonal antibodies (mAbs) and antigens are coated to detect SARS-CoV-2 antigens and antibodies. Therefore, NasRED refers to a more general design concept that allows different antigen or antibody detection, and Nano2RED is a special case where designed nanobinders are used for protein detection.
Simple, portable, and inexpensive readout instrument.
Recently, we designed an inexpensive, portable, electronic detection (PED) device based on semiconductor components, such as LEDs, photodetectors, transistors, resistors, and capacitors, with signal-stabilizing mechanism on an integrated printed circuit board (PCB). This new PED readout system, as small as <300 cm3 and costing <~$30 for materials, was demonstrated effective to minimize signal fluctuations for accurate detection at ultralow antigen and antibody concentrations. We are currently working to incorporate a multifunctional circuitry into the diagnostic system to allow direct data processing and wireless data transfer. We envision that the entire workstation will have the size of a laptop and cost <$1200.

Unique capability to achieve high sensitivity at a high speed
Conventional assays (such as ELISA) require passive surface incubation of the capturing agent (e.g., antibody) for antigen recognition. Since the diffusion velocity of molecules is very slow at the surface, long incubation time and multiple washing steps are needed to maximize the signal to noise ratio for a high sensitivity. In contrast, Nano2RED/NasRED is an in-solution assay without a stationary reaction surface. Antigen or antibody detection using the Nano2RED/NasRED platform is rate-limited by the sedimentation of AuNPs, which is influenced by the competition between the gravitational force and the fluidic drag force. The centrifugation step in Nano2RED simultaneously localizes the reagents at the tube bottom to boost low-concentration detection and greatly speed up signal transduction by reducing the diffusion length from millimeter to micrometer scale. As a result, Nano2RED allows a highly sensitive sample-to-readout within 15 to 30 minutes.
High analytical sensitivity:
Nano2RED enables high sensitivity antigen detection in complex physiological fluids. Previously we have shown successful detection of Ebola secreted glycoprotein (sGP) and SARS-CoV-2 spike protein receptor binding domain (RBD) with limit of detection (LoD) of ~10 to 40 pg/mL (0.13 pM), as well as a large dynamic range (~7 logs). Very recently, we showed that by further optimizing the sensing protocol and circuit design the LoD was improved to low-aM in diluted serum and whole blood for African swine fever, SARS-CoV-2 antibodies and Shiga toxin-producing E. coli, which are a few orders of magnitude better than ELISA.
For example, using AuNPs coated with the receptor binding domain (RBD) protein, NasRED was able to detect SARS-CoV-2 antibody AS35 in PBS with a limit of detection (LoD) of ~66 aM (~10 fg/mL) and a dynamic range of about 9 logs. Additionally, using two sets of AuNPs coated with different mAbs – AS47 and AM223 – that cooperatively target distant epitopes of the SARS-CoV-2 N-protein, Nano2RED achieved a LoD of ~470 aM (~54 fg/mL) in PBS. Lastly, the LoDs of AS35 antibody are 76 aM (~11 f/mL) in serum and 700 aM (~105 fg/mL) in 20% whole blood, while the LoDs of N-protein are 870 aM (~100 fg/mL) in saliva and 4.1 fM (~470 fg/mL) in nasal fluid.
Small sample volume and low testing cost.
Nano2RED does not require any labeling/washing or enzymatic reaction, therefore reducing the number of processing steps and minimizing sample volume and reagent usage. Since the sample volume (4 to 8 µL) is much smaller than a single drop of blood (~30 µL), Nano2RED is ideal for testing from capillary blood. The test cost is mainly dependent on the use AuNPs, which costs ~$0.1 per µL, or ~$2 per test (20 µL).
Reference:
- Yeji Choi†, Seyedsina Mirjalili†, Md Ashif Ikbal, Sean McClure, Scott Clemens, Jose Solano, John Heggland, Jiawei Zuo, and Chao Wang*, “Portable, Rapid, and Digital Detection of SARS-CoV-2 Antibodies and Antigens at Attomolar Level,” Under review. https://doi.org/10.1101/2024.09.04.611305

Personalized immunity assessment
Following viral infection, most individuals will develop an immune response to the virus, including the production of neutralizing antibodies (nAbs) that can prevent future infection. Therefore, it is necessary to quantify the levels of nAbs for vaccine development and personalized immunity evaluation. The gold standard for nAb analysis is the viral plaque reduction neutralization assay where viruses replicate inside cells grown in cultures and are subsequently released. This assay measures the titer of the specific antibody, however, it is not perfectly quantitative, very labour-intensive and time-consuming (a few days), and facility-demanding (BSL3 labs). We are currently funded by a NIH R21 grant to establish an easy-to-use, inexpensive and digital neutralizing assay to detect the neutralizing potency of monoclonal antibodies (from Prof. Michael Diamond from Washington University at St Louis) and collected human sera (from Dr. Vel Murugan at ASU) against the original SARS-CoV-2 Wuhan strain and Omicron variant. Our assay can be easily adapted to detect nAbs to almost any epitope in a variety of pathogens by exchanging the antigenic target. The low cost, simple operation, and automation capability are also very useful in longitudinal studies of the immune response related to COVID-19 or other infections. It can be used for determining the level of vaccination-elicited immunity against any emerging virus strains for special population (e.g. seniors in long-term care facilities or immune-compromised individuals).
Ongoing and future research activities
We aim to develop our technology to be deployed in the field and next to the patients with reduced health care cost and make it available in low- and middle-income countries. Currently, we are also funded by USDA (~$744k in 4 years) to apply our digital sensing technology for detection of African swine fever (ASF), a highly contagious and deadly disease in pigs that has no cure. In collaboration with a Spanish lab specialized in ASF, we have proven the feasibility of detection ASF antigens and antibodies in porcine sera, and are working to prove its feasibility using ASF reference samples. In the next few years, we expect to expand this digital sensing platform to other diseases such as hepatitis B/C, Lyme disease, autoimmune diseases, sexually transmitted diseases (HIV, Syphilis, etc.), bacterial infections, and cancer. We also expect to accelerate the translation of this technology to the market to tackle infectious disease, cardiovascular diseases, cancer, and neurodegenerative diseases.